People always knew there was light, but they didn't know what it was. Staying in dark rooms or tents, they saw it penetrate through narrow holes and light up suspended dust, and by that they knew it was spreading in a straight line. Only when innovative entrepreneurs began to produce precision and highly transparent lenses, prisms and mirrors did light exploration become possible.
The first theory about the nature of light was devised by Isaac Newton in 1672 when he assumed that light was consists of small particles moving at some speed. In doing so, he explained the linear motion of light, the occurrence of light refraction (refraction) and light reflection (reflection).
The further development of innovations devised various instruments, tools and procedures that enabled the further development of scientific experimentation. Experiments have thus found that light exhibits the properties of deflection, interference, and polarization, which could not be explained by particle theory.
In 1678, Christiaan Huygens elaborated and in 1690 published a new wave theory of light that better explained these phenomena. In 1850, Frenchman L. Foucault made accurate measurements of the speed of light in water using rotating mirrors and found that it was ¾ of the speed of light in a vacuum. This concludes that the wave theory is more accurate. According to wave theory, light waves propagate through the ether, fluid filling the entire space. Over time, physicists realized that this fluid could be millions of times stiffer than steel so it could support high frequencies of light, massless, completely transparent, non-dispersive, non-compressible, continuous and completely non-viscous.
In 1845, Mishael Faradey discovered that the angles of polarization of a light beam could be varied by the action of a magnetic field. In doing so, he conducted the connection between light and electromagnetism, and in 1847 he argued that light is a high-frequency electromagnetic vibration that can propagate along the media in the complete absence of something resembling Ether.
In 1887, Heinrich Herz observed that electrodes could easily create a spark if illuminated by ultraviolet radiation. Experiments have shown that this photoelectric effect is a physical phenomenon in which electrons erupt from irradiated material by electromagnetic radiation. This makes these materials electrically positive, since they have a shortage of electrically negative electrons. How strong this effect will be depends on the frequency of light, with only visible light radiation and ultraviolet radiation being able to eject electrons from metals.
In 1900, when studying Black's radiation energies, Max Planck assumed that the energy of a harmonic oscillator could receive only certain amounts, which he called Quant. According to him, the quantum of energy is the smallest indivisible amount of energy that can be emitted or absorbed by a quantum system, that is, an atomic nucleus, atom, or molecule. Before it, it was thought that in all physical phenomena energy transferred from one body to another continuously, in any small amount.
Since neither wave nor particle theory could explain all the experimentally determined phenomena, Albert Einstein published in 1905 his Special Theory of Relativity explaining the photo effect by introducing precisely certain amounts of energy or quantum of light, which he called a photon. Based on Planck's law, it is concluded that the amount of light quantum should be proportional to the frequency of light and multiplied by a constant, which was obtained by experiments as Planck's constant. The photo effect only occurred if a certain cutoff frequency was exceeded. Because of this discovery, A. Enstein reinstated the corpuscular theory in 1905, and determined that light is a swarm of particles, photons, which have energy dependent on Planck's constant and frequency. According to this, photons are particles of light that have a mass of zero and propagate at the speed of light. Einstein manages to explain these contradictions by introducing photons, particles of light that are an indivisible packet of energy. Now light is a swarm of photons, and when an electron absorbs a photon it only exits the metal if its energy is greater than or equal to the energy that the metal binds to it and does not allow it to leave.
Albert Einstein published the theory of general relativity in 1916. With this theory, he further relativistically Newton's theories of gravity, and in order to explain what he could not, he relativized gravity, space and time. He claimed that the speed of light is constant and that nothing can go faster than the speed of light, and that at that speed time stands still and space is curved. Since it was later implemented that some particles move faster than the speed of light and its claims were later abandoned. However, even today, many believe that at the speed of light, space is curved, that time stops, and that it is possible to travel through time.
Luis de Broglie envisioned electrons as having wave properties (dualism) in 1924, and by 1928 studied the arrangement of electrons in an atom. Apart from him, Erwin Schrodinger, Wolfgang Pauli, Max Born and Werner Heisenberg work on this theory. In 1923, Louis de Broglie proposed a theory of the dual property of light, extending it to matter in general, which he demonstrated by observing electron deflection on a crystal lattice. An electron, which is a particle, has a wavelength along it, which is a characteristic of a wave, so according to it, every body, because it is made up of particles, has a wavelength.
The quantum theory of light has somewhat explained the dualistic nature of light, which is manifested in the phenomena of interference and photoelectric effect. According to this theory, light is generated by quantum electron transitions from one energy state to another, in an atom or in a crystal lattice. Electrons in atoms are arranged by states of a certain energy (quantum states) and while they are in these states, they do not emit energy. When an electron is transferred to a quantum state of lower energy, the difference in energy is emitted as a quantum of electromagnetic radiation.
The dual theory has rather convincingly explained the physical properties of electromagnetic radiation (photons) and the basic particles of matter to exhibit both wave and particle properties, depending on the circumstances. When electromagnetic radiation spreads through space, deflection and interference occur, which is the evidence that it has wave properties. When electromagnetic radiation interacts with electrons from a substance in a photoelectric effect, it behaves like a swarm of tiny indivisible particles, lumps of energy called photons. In one circumstance (interaction with electrons in metal) electromagnetic radiation acts as a swarm of particles, and in other circumstances (propagation by space) as a wave. However, even these theories failed to objasniti all the facts.
The basic problem with all these theories is that they do not capture the complete nature of light, which is best explained by particle array theory.
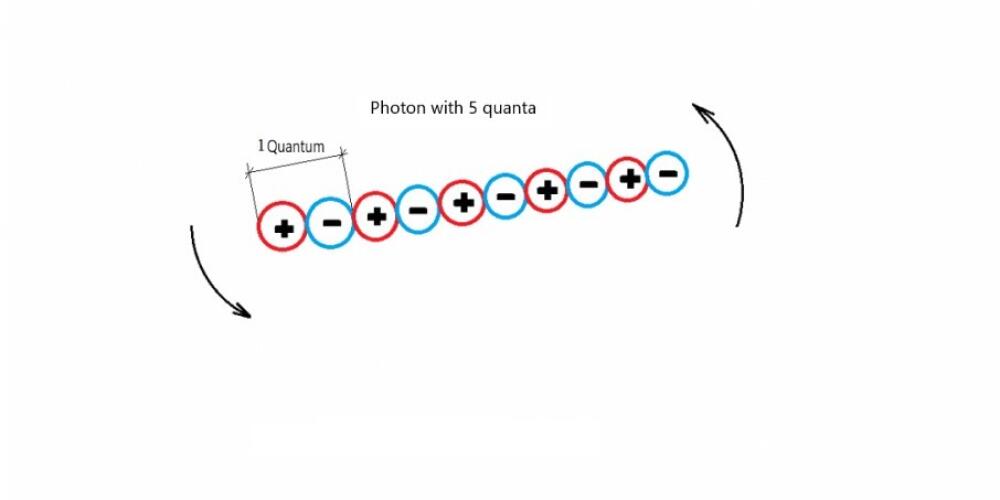
It is true that light is a particle (photon), but it is incorrect that light is also a wave. The wave properties of light are due to the shape of the photon. Light particles consist of even smaller, electrically charged positive and negative particles, which are alternately arranged in long thin series called photons, and one pair of these particles form one quantum. These arrays rotate like a rod when thrown into the air, creating a rotating magnetic field, which is reflected as the frequency of electromagnetic radiation. Long strings rotate more slowly and short strings faster. A photon is formed in an atom, upon cooling of an atom, when part of the electrical particles from electrons and protons are separated, by the action of an electric attracting force, they alternately interconnect and form a series. They alternate because same charge particles are rejected and the opposite of charge particles are attracted. In order for this array to leave the atom, it must attain a (minimum) rate known as the speed of light, which is the rate at which the photon leaves the atom.
As long as electrons and protons have an electric charge, ie, electrical particles in their composition can eject photons. When electrons and protons remain free of electrical charge, ie without electrical particles, the atom is cooled to absolute zero and can no longer eject photons.
Photons coming into an atom decompose into constituents. The positive parts go toward protons and the negative ones toward electrons, and the temperature of the atom rises. When passing through some substances, the photon can be decomposed into two photons, with the two photons having a smaller number of quanta, which in total equals the quantum number of photons from which they were generated. If a substance through which photons pass through absorbs photons then that substance can eject its photons of some other wavelength.
This particle array theory explains all the wave, particle, and electrical properties of light, and will one day cause all other light theories to be abandoned.
Tags
Featured articles